Answer: The amount of oxygen gas consumed by mouse is 0.202 grams.
Step-by-step explanation:
We are given:
Initial pressure of air = 769.0 torr
Final pressure of air = 717.1 torr
Pressure of oxygen = Pressure decreased = Initial pressure - Final pressure = (769.0 - 717.1) torr = 51.9 torr
To calculate the amount of oxygen gas consumed, we use the equation given by ideal gas which follows:

where,
P = pressure of the gas = 51.9 torr
V = Volume of the gas = 2.20 L
T = Temperature of the gas = 292 K
R = Gas constant =
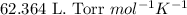
n = number of moles of oxygen gas = ?
Putting values in above equation, we get:
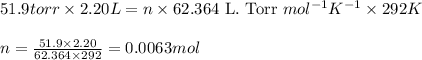
To calculate the mass from given number of moles, we use the equation:

Moles of oxygen gas = 0.0063 moles
Molar mass of oxygen gas = 32 g/mol
Putting values in above equation, we get:

Hence, the amount of oxygen gas consumed by mouse is 0.202 grams.