Answer:
-64 kJ/mol
Step-by-step explanation:
For the reaction:
AgNO₃ + NaCl → AgCl + NaNO₃
The heat of calorimeter is:
105,5 J/°C × (25,16°C - 24,69°C) = 49,59 J
The total volume of the solutions is 200,0mL + 100,0mL = 300,0mL ≡ 300,0g. Using this value, the heat generated in the water is:
4,184J/g°C × 300,0g × (25,16°C - 24,69°C) = 589,9J
As the heat of reaction is:
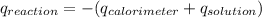

-639,5 J
As moles of AgCl are 0,1L × 0,100M (NaCl solution, limiting reagent) = 0,01 moles, ΔH reaction is:
-639,5 J / 0,01 moles = -63950 J/mol ≡ -64 kJ/mol
I hope it helps!