Answer:
0.17%
Step-by-step explanation:
With the equation:
2Cr2O7 2- + C2H5OH + H2O --> 4Cr3+ + 2CO2 + 11H2O
We can assume that every mole of ethanol needs 2 moles of Dichromate to react.
So if in 1L we have 0.05961 moles of dichromate we can discover how many moles we have in 35.46mL
1000 mL - 0.05962 moles
35.46 mL - x
x =
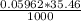
x = 2,11* 10^-3 moles
As we said earlier, 1 mole of ethanol needs 2 mole of dichromate, so in the solution we have 1,055*10^-3 moles of ethanol. We can discover the mass of ethanol present in the solution.
1 mole - 46g
1.055*10^-3 - y
y = 46 * 1.055*10^-3
y = 0.048 g
To discover the percent of alchol we can use a simple relation
28 g - 100%
0.048 - z
z =
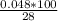
z = 0.17%