Answer:
16,165 ton
Step-by-step explanation:
120.91 g - 1 mole of CFC-12
1100 g - x mole of CFC-12
x =
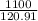
x = 9,10 moles
9,10 moles - 100%
y - 25% that leakes
y =
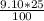
y = 2.28 mole
A mole of chlorine has 35,45 g, so 2 moles of chlorine has 70,90 g
1 mole of CFC-12 - 70,90 g
2.28 moles of CFC-12 - z
z = 70.90 * 2.28
z = 161,65 g of chlorine per air conditioner.
For 100 million air conditioner
t = 161.65 g * 100 * 10^6
t = 16,165 ton