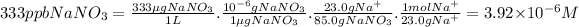Answer:
Concentration of Hg = 0.5 ppm
Concentration of Na⁺ = 3.92 × 10⁻⁶ M
Step-by-step explanation:
The level of Hg is 0.5 mg Hg per kg tissue. What is this level in ppm?
Depending on the context, ppm may refer to mg/kg (ppm in mass) or mg/L(ppm in volume). In this case, we assume it is ppm in mass. Since the level of Hg is 0.5 mg/kg, it is the same as 0.5 ppm.
What is the molarity of Na+ in an aqueous solution containing 333 ppb of NaNO₃?
Depending on the context, ppm may refer to μg/kg (ppb in mass) or μg/L(ppb in volume). In this case, we assume it is ppb in mass. We can find the molarity of Na⁺ (moles of Na⁺ per liter of solution) using proportions.