Answer:
a) 0,5 mol O₂; 1 mol NO₂
b)
Liters of NO = 22,4 L
Liters of O₂ = 11,2 L
Liters of NO₂ = 22,4 L
c) NO = 30g
O₂ = 16g
NO₂ = 46g
d) 7,41g HCl
e) 107,7 g/mol
f) 0,0312 moles of O₂; 0,999g of O₂; 699 mL at STP or 803 mL
ii. 51%
g) 32,6L
Step-by-step explanation:
a) For the reaction:
2 NO + O₂ → 2 NO₂
For 1 mole of NO there are consumed:
1 mol NO ×
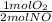 = 0,5 mol of O₂
= 0,5 mol of O₂
And produced:
1 mol NO ×
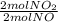 = 1 mol of NO₂
= 1 mol of NO₂
b) By ideal gas law:
V = nRT/P
Where n is moles of each compound; R is gas constant (0,082atmL/molK); T is temperature (273,15 K at state conditions); P is pressure (1 atm at STP) and V is volume in liters. Replacing each moles for each compound:
Liters of NO = 22,4 L
Liters of O₂ = 11,2 L
Liters of NO₂ = 22,4 L
c) The mass of each compound are:
1 mol NO×
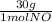 = 30g
= 30g
0,5 mol O₂×
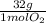 = 16g
= 16g
1 mol NO₂×
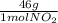 = 46g
= 46g
d) Using:
n = PV / RT
Moles of 4,55 L of HCl (using the values of P = 1 atm; R = 0,082atmL/molK; T = 273,15K) are:
0,203 moles of HCl. In grams:
0,203 mol HCl×
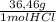 = 7,41 g of HCl
= 7,41 g of HCl
e) Using:
δRT/P = MW
Where δ is density in g/L (4,81 g/L); R is gas constant (0,082atmL/molK); T is temperature (273,15K); P is pressure (1 atm)
And MW is molecular mass: 107,7 g/mol
f) For the reaction:
2 KClO₃ → 2 KCl + 3 O₂
2,550 g of KClO₃ are:
2,550 g of KClO₃×
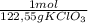 = 0,0208 moles of KClO₃
= 0,0208 moles of KClO₃
When these moles reacts completely produce:
0,0208 moles of KClO₃×
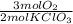 = 0,0312 moles of O₂
= 0,0312 moles of O₂
In grams:
0,0312 moles of O₂ ×
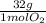 = 0,999g of O₂
= 0,999g of O₂
V = nRT/P
At STP, n = 0,0312 mol; R = 0,082atmL/molK;T= 273,15K; P = 1atm; V = 0,699L ≡ 699mL
At 29 °C (302,15K) and 732 torr (0,963 atm)
V = 0,803L ≡ 803mL
ii. 182 mL ≡ 0,182L of O₂ are:
n = PV/RT
moles of O₂ are 7,07x10⁻³. Moles of KClO₃ are:
7,07x10⁻³ moles of O₂×
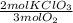 = 0,0106 mol KClO₃. In grams:
= 0,0106 mol KClO₃. In grams:
0,0106 moles of KClO₃×
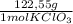 = 1,300 g of KClO₃.
= 1,300 g of KClO₃.
Thus, percent by mass of KClO₃ in the mixture is:
1,300g/2,550g ×100 = 51%
g. Combined gas law says that:
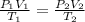
Where:
P₁ = 755 torr; V₁ = 35,9L; T₁ = 26°C (299,15 K); P₂ = 760 torr (STP): T₂ = 273,15K (STP) V₂ = 32,6 L
I hope it helps!