Answer:
Δ
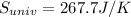
Step-by-step explanation:
Hello,
To find the change in the entropy of the universe, we must take into account the following entropy balance:
Δ
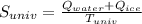
We can stand the universe as the surroundings so the
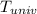 equals the outside air temperature. Then, we must compute both the water's and ice's released heat due to their interaction with the "hot" air:
equals the outside air temperature. Then, we must compute both the water's and ice's released heat due to their interaction with the "hot" air:
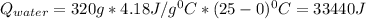
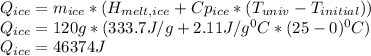
Finally, we obtain the change (increasing) in the entropy of the universe as follows:
Δ
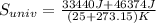
Δ
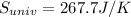
* All the data were extracted from Cengel's thermodynamics book.
Best regards.