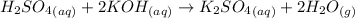Answer:
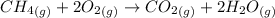
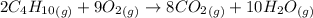
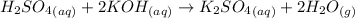
Step-by-step explanation:
(a)
The balanced chemical equation for the reaction of methane with oxygen gas to form carbon dioxide and water is shown below as;
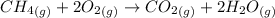
(b)
The balanced chemical equation for the reaction of butane with oxygen gas to form carbon dioxide and water is shown below as;
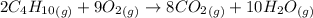
(c)
The balanced chemical equation for the reaction of solution of sulfuric acid reacts with aqueous potassium hydroxide to produce potassium sulfate and water is shown below as;