Answer:
2.1
Step-by-step explanation:
Calculation of moles of
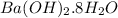
Mass of copper = 5.3 g
Molar mass of copper = 315.46 g/mol
The formula for the calculation of moles is shown below:

Thus,
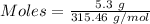
Moles of
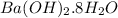 = 0.0168 moles
= 0.0168 moles
According to the reaction,
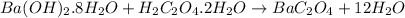
1 mole of
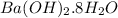 react with 1 mole of
react with 1 mole of
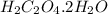
0.0168 moles of
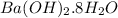 react with 0.0168 moles of
react with 0.0168 moles of
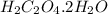
Moles of
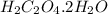 = 0.0168 moles
= 0.0168 moles
Molar mass of
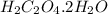 = 126.07 g/mol
= 126.07 g/mol
Thus,
Mass = Moles * Molar mass = 0.0168 moles * 126.07 g/mol = 2.1 g
Answer - 2.1