Answer:
The moles of A⁻ present in the mixture are 3,20x10⁻⁵ in a format 3,20E-5
Step-by-step explanation:
A buffer is a mixture of a weak acid (In this case, HA) with its conjugate base.
The moles of weak acid are:
5,00 mL×
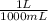 = 5x10⁻³ L of HA
= 5x10⁻³ L of HA
5x10⁻³ L of HA×
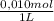 = 5x10⁻⁵ mol of HA
= 5x10⁻⁵ mol of HA
The initial moles of conjugate base are:
3,00 mL×
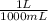 = 3x10⁻³ L of NaA
= 3x10⁻³ L of NaA
3x10⁻³ L of HA×
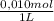 = 3x10⁻⁵ mol of NaA
= 3x10⁻⁵ mol of NaA
The reaction of a weak acid with a strong base as NaOH produce:
HA + NaOH → NaA + H₂O
0.20 mL 0.010 M NaOH are:
0,20 mL×
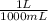 = 2x10⁻⁴ L of HA
= 2x10⁻⁴ L of HA
2x10⁻⁴ L of HA×
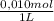 = 2x10⁻⁶ mol of NaOH
= 2x10⁻⁶ mol of NaOH
Each mol of NaOH is producing NaA. That means:
2x10⁻⁶ mol of NaOH ≡ 2x10⁻⁶ mol of NaA
Thus, moles of A⁻ present in the mixture are:
2x10⁻⁶ mol of NaA + 3x10⁻⁵ mol of NaA = 3,20x10⁻⁵ mol of NaA, in E-format: 3,20E-5
I hope it helps!