Answer:
1356.33 g/mol
Step-by-step explanation:
Number of hydrogen in 1 molecule of cyanocobalamin = 88
Molar mass of hydrogen = 1.008 g/mol
So, Mass of hydrogen in one mole of cyanocobalamin = 88*1.008 = 88.704 g
Also, Given that mass of hydrogen is 6.54 % Of the mass of cyanocobalamin.
Thus,
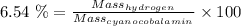
So, Applying values, we get:
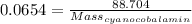
Mass of 1 mole of cyanocobalamin = 1356.33 g
So, molar mass = 1356.33 g/mol