Answer:
(C) bent 109
(2)
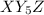
Step-by-step explanation:
The number of bond pairs = 2
Number of lone pairs = 2
The general formula is -
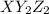
According to VSEPR theory, the atoms will form a geometry in such a way that there is minimum repulsion and maximum stability.
The central atom has 2 bond pairs and 2 lone pairs. Hybridization is sp³. The geometry is bent shape which has an angle of approximately 105.5°. It original has angle of 109.5° but due to lone pair repulsion, it reduces.
Answer - (C) bent 109
Square pyramidal possibility:- (2)
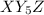
The central atom has 5 bond pairs and 1 lone pair. Hybridization is sp³d. The geometry is square pyramidal in which the equatorial bonds has an angle of 120° and axial bond has an angle of 90°.