Answer:
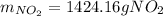
Step-by-step explanation:
Hello,
At first, the combustion of octane is illustrated as shown below:

Now, since 800 grams of octane are burned, we compute the moles of oxygen that reacted via stoichiometry:
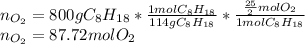
As that is the combusting oxygen, we now look for the total oxygen that was employed by:
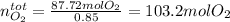
Therefore the 15% used in the NO₂ turns out:
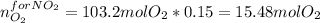
Finally, for the NO₂ production:
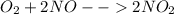
The produced grams of NO₂ are:
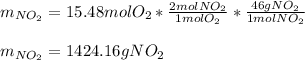
Best regards.