Answer: The mass of KI present in 1 kg of solution is 25 grams.
Step-by-step explanation:
We are given:
2.5 % (w/w) of KI solution.
This means that 2.5 grams of KI is present in 100 grams of solution.
We need to find the mass of KI present in 1 kg of solution, we use the conversion factor:
1 kg = 1000 g
Calculating the mass of KI in 1 kg or 1000 g of solution by unitary method:
In 100 grams of solution, mass of KI present is 2.5 grams
So, in 1000 grams of solution, mass of KI present will be =
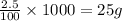
Hence, the mass of KI present in 1 kg of solution is 25 grams.