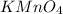Answer:
C)
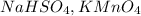
Step-by-step explanation:
- For the sodium hydrogen sulfate:
The ending -ate of the word sulfate indicates that the compound comes from the ion sulfate that is
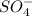 , so the compound formed by this ion will be
, so the compound formed by this ion will be
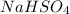
- For the potassium permanganate:
The ending -ate of the word permanganate indicates that the compound comes from the ion
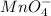 , so the compound formed by this ion will be
, so the compound formed by this ion will be