Answer: The volume of ammonia required from first solution to make second solution is 5.72 mL
Step-by-step explanation:
To calculate the molarity of the diluted solution, we use the equation:
 .......(1)
.......(1)
where,
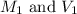 are the molarity and volume of the concentrated ammonia solution
are the molarity and volume of the concentrated ammonia solution
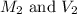 are the molarity and volume of diluted ammonia solution
are the molarity and volume of diluted ammonia solution
We are given:
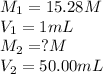
Putting values in equation 1, we get:
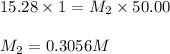
Now, calculating the volume of ammonia diluted from first dilution to make the required ammonia solution by using equation 1:
We are given:
 = Molarity of ammonia solution from first dilution = 0.3056 M
= Molarity of ammonia solution from first dilution = 0.3056 M
 = Volume of ammonia solution from first dilution = ?
= Volume of ammonia solution from first dilution = ?
 = Molarity of ammonia to make second solution = 0.0350 M
= Molarity of ammonia to make second solution = 0.0350 M
 = Volume of ammonia to make second solution = 50.00 mL
= Volume of ammonia to make second solution = 50.00 mL
Putting values in equation 1, we get:
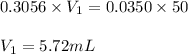
Hence, the volume of ammonia required from first solution to make second solution is 5.72 mL