Answer: The correct statements are adding more
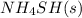 to the vessel and increasing the volume of the vessel
to the vessel and increasing the volume of the vessel
Step-by-step explanation:
For the given chemical reaction:
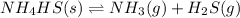
According to Le-Chateliers principle, if there is any change in the variables of the reaction, the equilibrium will shift in the direction in order to minimize the effect.
The given statements are:
- Statement 1: Adding more
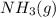 to the vessel
to the vessel
When ammonia gas is added, the concentration of products get increased. So, by Le-Chatelier's principle, the equilibrium will shift in the direction where concentration of products must decrease, which is in the backward direction.
Thus, concentration of
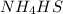 will increase.
will increase.
- Statement 2: Adding more
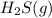 to the vessel
to the vessel
When hydrogen sulfide gas is added, the concentration of products get increased. So, by Le-Chatelier's principle, the equilibrium will shift in the direction where concentration of products must decrease, which is in the backward direction.
Thus, concentration of
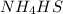 will increase.
will increase.
- Statement 3: Adding more
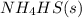 to the vessel
to the vessel
When
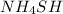 is added, the concentration of reactants get increased. So, by Le-Chatelier's principle, the equilibrium will shift in the direction where concentration of reactants must decrease, which is in the forward direction.
is added, the concentration of reactants get increased. So, by Le-Chatelier's principle, the equilibrium will shift in the direction where concentration of reactants must decrease, which is in the forward direction.
Thus, concentration of
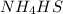 will decrease.
will decrease.
- Statement 4: Increasing the volume of vessel
When volume is increased, the equilibrium will shift in the direction which produces more moles of gas.
In the given reaction, number of moles of gas is more on the product side. So, the reaction will move towards forward direction.
Thus, concentration of
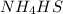 will decrease.
will decrease.
- Statement 5: Decreasing the volume of vessel
When volume of the vessel is decreased, the equilibrium will shift in the direction which produces fewer moles of gas.
In the given reaction, number of moles of gas is less on the reactant side. So, the reaction will move towards backward direction.
Thus, concentration of
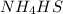 will increase.
will increase.
Hence, the correct statements are adding more
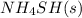 to the vessel and increasing the volume of the vessel
to the vessel and increasing the volume of the vessel