Answer:
5.4 * 10⁵ L
Step-by-step explanation:
The series of reactions is:
- 3C₂H₄ + 2H₂SO₄ → C₂H₅HSO₄ + (C₂H₅)₂SO₄
- C₂H₅HSO₄ + (C₂H₅)₂SO₄ + 3H₂O → 3C₂H₅OH + 2H₂SO₄
We can see that both ethylene (C₂H₄) and ethanol (C₂H₅OH) have a stoichiometric coefficient of 3. In other words, one mol of ethylene equals 1 mol of ethanol.
Now we calculate the moles of ethanol that we want to produce:
- 1000 kg = 1000 * 10³ g = 10⁶ g
10⁶ g ethanol ÷ 46 g/mol = 2.1739 * 10⁴ mol ethanol
Calculating the moles of ethylene required, keeping in mind the yield:
2.174 * 10⁴ mol ethanol *
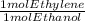 * 100/90.1 = 2.4128* 10⁴ mol ethylene
* 100/90.1 = 2.4128* 10⁴ mol ethylene
Now we use PV=nRT to calculate V, because ethylene is a gas at STP. Using STP means that we have T = 273 K, and P = 1 atm.
1 atm * V = 2.4128* 10⁴ * 0.082 atm·L·mol⁻¹·K⁻¹ * 273 K
V = 5.4 * 10⁵ L