Answer:
1. B) increase
2. B) decrease
3. C) increase.
4. B) decrease
Step-by-step explanation:
The ionic strength (I) of a solution depends on the concentration (ci) and charge (zi) of the ion in the solution. The higher the concentration and charge of the ions the greater the ionic force.
I = 1/2 ∑
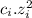
1. If a solution of HNO3 were added to a solution of KOH, the ionic strength of the KOH solution would: B) increase
Because the final solution has H+, NO3-, K+ and OH-, thus a higher concentration of ions.
2. If a dilute solution of KOH were added to a solution of CaCl2 (Ca(OH)2(s) is formed), the ionic strength of the CaCl2 solution would: B) decrease
Because the Calcium cation has a charge of +2, and the formation of Ca(OH)2(s) removes the Ca+2 in the solution and it is replace for the Potassium cation that has a charge of +1. While the concentration of Cl- remains constant. Thus, the charge of the mixture is lower than the solution of CaCl2.
3. If a dilute solution of benzoic acid were titrated with a solution of KOH, the ionic strength of the benzoic acid solution would: C) increase.
Because a solution of benzoic acid is partially ionized but after a titration all the concentration of benzoic acid turns into benzoate and there is also K+ cations.
4. If a solution of NaOH were added to a solution of iron(II) chloride, the ionic strength of the iron(II) chloride solution would: B) decrease
In basic conditions iron(II) chloride it oxidizes into iron(III) chloride and this precipitates as Fe(OH)3(s). Thus, the initial solution has Fe+2 which is replaced in the mixture by K+. While the concentration of Cl- remains constant. Hence the charge of the mixture is lower than the initial solution.