Answer:
The chemist needs to add 400mL of 40% solution.
Explanation:
The equation
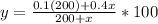
gives the percent concentration
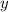 of the final mixture, when
of the final mixture, when
 mL of the 40% solution are added.
mL of the 40% solution are added.
Now we are asked, how many milliliters
 of the 40% solution should the chemist add to get final percent concentration
of the 40% solution should the chemist add to get final percent concentration
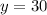 ; this is just a matter of solving the equation
; this is just a matter of solving the equation
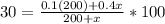
and we solve it the following way:
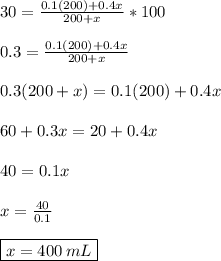
Thus, the chemist needs 400mL of 40% solution to get 30% concentration of the final mixture.