Step-by-step explanation:
The given reaction is as follows.
![E + S \rightleftharpoons ES \xrightarrow[]{k_(2)} E + P](https://img.qammunity.org/2020/formulas/chemistry/college/fb56fw90lhaquth65mysaswwpt73ng9k3r.png)
Here, [E] = triose phosphate isomerase = 0.1
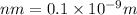
[S] = Dihydroxy acetone phosphate = 5
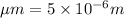
[P] = Glyceraldehyde-3-phosphate = 2
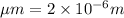
Therefore, velocity of the reaction will be as follows.
v =
![(d[P])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/college/bmpkha3l1p5e5r710pn2yd7i578d4psdlk.png) =
=
![(K_(2)[E][S])/(K_(M) + [S])](https://img.qammunity.org/2020/formulas/chemistry/college/v16wrx86o9kn3zskhj8li46gwcuv1wxoc7.png)
where,
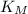 = Michaelic menten constant =
= Michaelic menten constant =
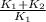
v =
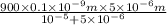
=
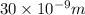
or, = 30 nm/s
Hence, we can conclude that the actual velocity of the forward reaction under physiologic conditions if KM = 10 μM is 30 nm/s.