Answer:
13.92 %
Step-by-step explanation:
Mass of
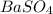 = 12.5221 g
= 12.5221 g
Molar mass of
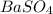 = 233.43 g/mol
= 233.43 g/mol
The formula for the calculation of moles is shown below:

Thus,
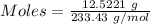
Moles of
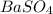 = 0.0536 moles
= 0.0536 moles
According to the given reaction,
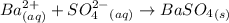
1 mole of
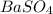 is formed from 1 mole of
is formed from 1 mole of
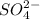
Thus,
0.0536 moles of
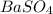 is formed from 0.0536 moles of
is formed from 0.0536 moles of
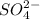
Moles of
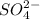 = 0.0536 moles
= 0.0536 moles
Moles of sulfur in 1 mole
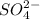 = 1 mole
= 1 mole
Moles of sulfur in 0.0536 mole
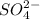 = 0.0536 mole
= 0.0536 mole
Molar mass of sulfur = 32.065 g/mol
Mass = Moles * Molar mass = 0.0536 * 32.065 g = 1.7187 g
Mass of ore = 12.3430 g
Mass % =
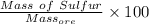 =
=
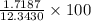 = 13.92 %
= 13.92 %