Answer:
Approximately 0.126 M
Step-by-step explanation:
For the calculation of the dilution you take into account the moles of NaOH in the 42.1mL of the original solution and you use the new volume of 342.1 mL:
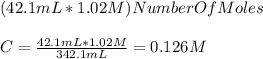
The standardization is necessary because a beaker is not not an instrument used to measure volumes and the marks on it only give an estimate of the volume of the solution, they are used to contain solutions and carry reactions among other things. If you would have measured the water with a graduated cylinder (an instrument designed to measure volumes) the standardization wouldnt be that necessary.