Answer:
1 atom of Cu has a mass of 1.055×
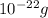
Step-by-step explanation:
1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step).
Avogadro's Number( 1 mole = 6.022 x
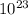 particles and the relative atomic mass of copper( 1 mole of copper = 63.546 g ) are the equalities required to make this conversion.
particles and the relative atomic mass of copper( 1 mole of copper = 63.546 g ) are the equalities required to make this conversion.