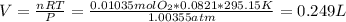Answer:
0.249 L O2 gas
Step-by-step explanation:
This a limit reagent problem, which means that you have to calcule how much product will form supposing a complete chemical reaction upon a limit of one reagent (the one you have the less quantity stoichiometrically).
To calcule the limit reagent you need to do your calculations in moles since a chemical reaction is expressed in moles of any given compound, so you calcule how many moles are in certain grams of MnO2 or KClO3 using their molecular weight. Also you divide the number of moles by the stoichiometric coefficient (given by the reaction) because you need to take into account how many moles of the compound are necessary for the reaction to continue.
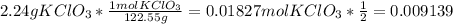
![0.30g MnO_(2) * (1 mol MnO_(2))/(86.936 g) =0.003451molMnO_(2)</p><p>Then the limit reagent is MnO2.</p><p>Afterwards you calcule how many moles of O2 are formed by the reaction of 0.30 g of MnO2:</p><p>[tex]0.30 gMnO_(2)*(1 mol MnO_(2))/(86.936gMnO_(2)) *(3mol O_(2) formed)/(1 mol MnO_(2)consumed)= 0.01035 mol O_(2)]()
Knowing the moles formed you can use the ideal gas law to calcule the volume of O2 in the given conditions: