Answer:
(a). The ground state energy is 16.56 eV.
(b). The wavelength of the emitted photon is 4.67 nm.
Step-by-step explanation:
Given that,
Size l= 0.15 nm
We need to calculate the ground state energy
Using formula of energy state
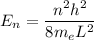
For ground state,
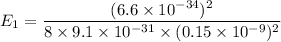
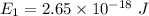
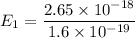
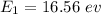
The ground state energy is 16.56 eV.
(b). We need to calculate the energy difference between 5th excited and 3rd excited state
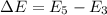
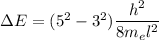
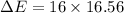
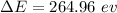
We need to calculate the wavelength of the emitted photon
Using formula of wave length
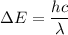
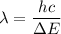
Put the value into the formula
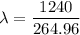
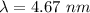
The wavelength of the emitted photon is 4.67 nm.
Hence, This is the required solution.