Answer: A. 23.59 minutes.
B. 249.65 minutes
Explanation: This question involves the concept of Latent Heat and specific heat capacities of water in solid phase.
Latent heat of fusion is the total amount of heat rejected from the unit mass of water at 0 degree Celsius to convert completely into ice of 0 degree Celsius (and the heat required for vice-versa process).
Specific heat capacity of a substance is the amount of heat required by the unit mass of a substance to raise its temperature by 1 kelvin.
Here, given that:
- temperature of ice, T= -16.6°C
- rate of heat transfer,
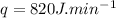
- specific heat of ice,
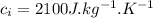
- latent heat of fusion of ice,
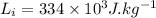
Asked:
1. Time require for the ice to start melting.
2. Time required to raise the temperature above freezing point.
Sol.: 1.
We have the formula:
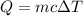
Using above equation we find the total heat required to bring the ice from -16.6°C to 0°C.
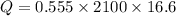
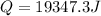
Now, we require 19347.3 joules of heat to bring the ice to 0°C and then on further addition of heat it starts melting.
∴The time required before the ice starts to melt is the time required to bring the ice to 0°C.
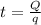
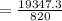
= 23.59 minutes.
Sol.: 2.
Next we need to find the time it takes before the temperature rises above freezing from the time when heating begins.
Now comes the concept of Latent heat into the play, the temperature does not starts rising for the ice as soon as it reaches at 0°C it takes significant amount of time to raise the temperature because the heat energy is being used to convert the phase of the water molecules from solid to liquid.
From the above solution we have concluded that 23.59 minutes is required for the given ice to come to 0°C, now we need some extra amount of energy to convert this ice to liquid water of 0°C.
We have the equation: latent heat,
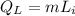
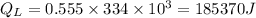
Now the time required for supply of 185370 J:
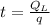
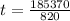
t= 226.06 minutes
∴ The time it takes before the temperature rises above freezing from the time when heating begins= 226.06 + 23.59
= 249.65 minutes