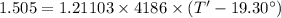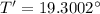Answer:
The temperature after half an hour is
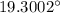
Solution:
As per the question;
Metabolic rate of the person is 3.0105 J/h
Temperature, T =
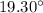
Mass of the water,
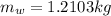
Time duration, t = 0.5 h = 30 min = 180 s
Now,
Heat,
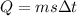
Thus heat transfer in half an hour:
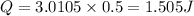
Now, the temperature of water after half an hour, T' is given by:
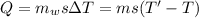
where
s = 4186 J