Answer: The mass of 0.5 mole of element ab is 81.67 g.
Step-by-step explanation:
According to the mole concept, 1 mole of a substance contains
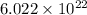 . As, the mass of
. As, the mass of
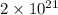 atoms is 0.49 g.
atoms is 0.49 g.
So,
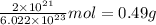
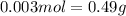
Therefore, mass of 1 mole =
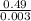
= 163.33 g/mol
Hence, mass of 0.5 mole of ab element is calculated as follows.
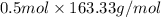
= 81.67 g
Therefore, the mass of 0.5 mole of element ab is 81.67 g.