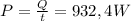Answer:
The minimum power neccesary is:
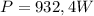
Step-by-step explanation:
The water is bring to a boil so it goes from 25°C to 100°C, the temperature rise si:
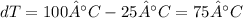
Considering that the cup is always at the same temperatura as the water the trasfered energy can be calculated as:
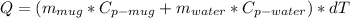
We have that:
![m_(mug)=0,25kg, C_(p-mug)=950 J/(kg.°C), m_(water)=0,30kg, C_(p-water)=4180 J/(kg.°C) (considering it constant)]()
So:
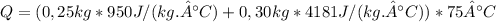
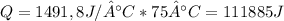
Given that this energy was supplied in 2 minutes:
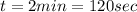
The minimum power neccesary is: