Answer : The pH of 0.289 M solution of lithium acetate at
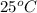 is 9.1
is 9.1
Explanation :
First we have to calculate the value of
 .
.
As we know that,
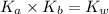
where,
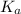 = dissociation constant of an acid =
= dissociation constant of an acid =
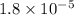
 = dissociation constant of a base = ?
= dissociation constant of a base = ?
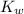 = dissociation constant of water =
= dissociation constant of water =
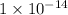
Now put all the given values in the above expression, we get the dissociation constant of a base.
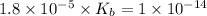
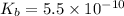
Now we have to calculate the concentration of hydroxide ion.
Formula used :
![[OH^-]=(K_b* C)^{(1)/(2)}](https://img.qammunity.org/2020/formulas/chemistry/high-school/cb0gsvyc1mib4nk1pon5yak5jjkkrlx0gl.png)
where,
C is the concentration of solution.
Now put all the given values in this formula, we get:
![[OH^-]=(5.5* 10^(-10)* 0.289)^{(1)/(2)}](https://img.qammunity.org/2020/formulas/chemistry/high-school/c45c1jf153z1qt29tlpkumjtjxrimfnz8d.png)
![[OH^-]=1.3* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/cuvpij0qs4ad8l4buhxy1jfpm4a2rz7qj5.png)
Now we have to calculate the pOH.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
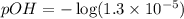
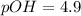
Now we have to calculate the pH.
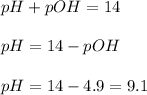
Therefore, the pH of 0.289 M solution of lithium acetate at
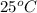 is 9.1
is 9.1