Answer:
Kp = 0.81666
Step-by-step explanation:
Pressure of PCl₅ = 0.500 atm
Considering the ICE table for the equilibrium as:
PCl₅ (g) ⇔ PCl₃ (g) + Cl₂ (g)
t = o 0.500
t = eq -x x x
--------------------------------------------- --------------------------
Moles at eq: 0.500-x x x
Given the pressure of PCl₅ at equilibrium = 0.150 atm
Thus, 0.500 - x = 0.150
x = 0.350 atm
The expression for the equilibrium constant is:
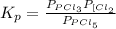
So,
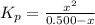
x = 0.350 atm
Thus,
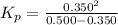
Thus, Kp = 0.81666