Step-by-step explanation:
As it is given that there are six electrons with four of them unpaired in the degenerate orbital.
As in a p-orbital there are only 3 sub-orbitals, that is,
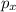 ,
,
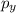 , and
, and
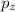 . In these sub-orbitals, a maximum of 6 electrons can be accommodated.
. In these sub-orbitals, a maximum of 6 electrons can be accommodated.
Th next higher orbital is d-orbital which can contain a maximum of 10 electrons. There are 5 degenerate orbitals present in a d-orbital.
So, a d-orbital can easily accommodate six electrons with four of them unpaired.
Thus, we can conclude that 5 degenerate orbitals are needed to contain six electrons with four of them unpaired.