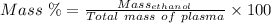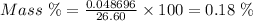Answer:
0.18 %
Step-by-step explanation:
Considering:

Or,
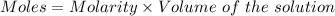
Given :
For
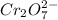 :
:
Molarity = 0.05961 M
Volume = 35.46 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 35.46×10⁻³ L
Thus, moles of
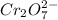 :
:
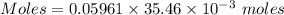
Moles of
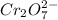 = 0.002114 moles
= 0.002114 moles
According to the given reaction:
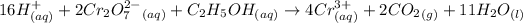
2 moles of
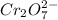 react with 1 mole of alcohol
react with 1 mole of alcohol
Thus,
0.002114 moles of
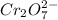 react with 1/2*0.002114 mole of alcohol
react with 1/2*0.002114 mole of alcohol
Moles of alcohol = 0.001057 moles
Molar mass of ethanol = 46.07 g/mol
Mass = Moles * Molar mass = 0.001057 * 46.07 g = 0.048696 g
Mass of plasma = 26.60 g
Mass Percent is the percentage by the mass of compound present in the mixture.