Answer:
A solution made by mixing 100 mL of 0.100 M HClO and 50 mL of 0.100 M NaOH.
Step-by-step explanation:
The options answers are:
A) A solution made by mixing 100 mL of 0.100 M HClO and 50 mL of 0.100 M NaOH.
B) A solution made by mixing 100 mL of 0.100 M HClO and 50 mL of 0.100 M HCl.
C) A solution made by mixing 100 mL of 0.100 M KClO and 50 mL of 0.100 M KCl.
D) A solution made by mixing 100 mL of 0.100 M HClO and 500 mL of 0.100 M NaOH.
We have to keep in mind that a buffer solution must contain and acid and this conjutaged acid in the same solution:
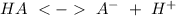
In option B we only have acids, HClO and HCl. Is not possible to have a buffer system with these compounds
In option C we have only salts KClO and KCl. Is not possible to have a buffer system with these compounds also.
For A and B options we have an acid HClO and a base NaOH. So, the conjugate base of the acid in these cases would be produced by the addition of NaOH. So, the question is which one of these possibilities is a real buffer system.
When we check the moles presents in D we find that we have 0.01 mol of HClO and 0.05 mol NaOH, so we will have an excess of base. This excess of base will convert all the acid into his conjugated base and we need both compounds in the buffer system, the acid, and the conjugated base. So, D is not an option.