Answer:
The feed ratio (liters 20% solution/liter 60% solution) is 3,08
Step-by-step explanation:
In this problem you have a 20,0 wt% H₂SO₄ and a 60,0 wt% H₂SO₄ solutions.
100 kg of 20% solution are 100kg/1,139 kg/L = 87,8 L
100kg×20wt% = 20 kg H₂SO₄. In moles:
20 kg H₂SO₄ × (1 kmol/98,08 kg) = 0,2039 kmol H₂SO₄≡ 203,9 mol
The final molarity 4,00M comes from:
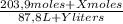 (1)
(1)
Where X moles and Y liters comes from 60,0 wt% H₂SO₄
100 L of 60,0 wt% H₂SO₄ are:
100L×
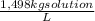 ×
×
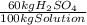 ×
×
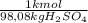 = 0,9164 kmolH₂SO₄ ≡ 916,4 moles
= 0,9164 kmolH₂SO₄ ≡ 916,4 moles
That means:
X/Y = 916,4/100 = 9,164 (2)
Replacing (2) in (1):
Y(liters of 60,0 wt% H₂SO₄) = 28,52 L
Thus, feed ratio (liters 20% solution/liter 60% solution):
87,8L/28,52L = 3,08
I hope it helps!