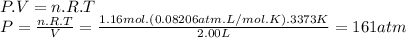Answer:
The pressure of acetylene should be 161 atm.
Step-by-step explanation:
Let's consider the reaction between oxygen and acetylene. This is a combustion reaction that produces carbon dioxide and water.
C₂H₂ + 2.5 O₂ ⇄ 2 CO₂ + H₂O
For O₂, we know it occupies 7.00 L and exerts a pressure of 115 atm. The welding with acetylene takes place at around 3373 K. In these conditions, we can use the ideal gas law to find out the amount of oxygen.
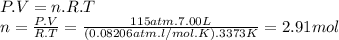
Using the balanced equation, we can calculate how much acetylene we need for 2.91 moles of O₂.
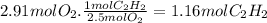
So, 1.16 moles of C₂H₂ occupy 2.00L at 3373 K. The pressure it exerts is: