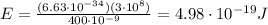Answer:
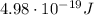
Step-by-step explanation:
The energy of the emitted photon is inversely proportional to its wavelength, according to the equation:

where
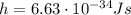 is the Planck's constant
is the Planck's constant
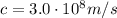 is the speed of light
is the speed of light
 is the wavelength
is the wavelength
This means that the biggest energy is released when the wavelength is the shortest. For a photon of visible light, the shortest wavelength is
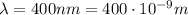
So, substituting into the equation, we find the corresponding energy: