Step-by-step explanation:
The given balanced reaction is as follows.
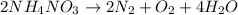
It is given that mass of ammonium nitrate is 86.0 kg.
As 1 kg = 1000 g. So, 86.0 kg = 86000 g.
Hence, moles of
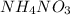 present will be as follows.
present will be as follows.
Moles of
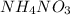 =
=
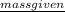
=
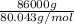
= 1074.42 mol
Therefore, moles of
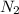 ,
,
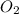 and
and
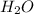 produced by 1074.42 mole of
produced by 1074.42 mole of
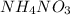 will be as follows.
will be as follows.
Moles of
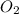 =
=
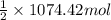
= 537.21 mol
Moles of
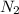 =
=
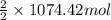
= 1074.42 mol
Moles of
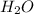 =
=
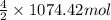
= 2148.84 mol
Therefore, total number of moles will be as follows.
537.21 mol + 1074.42 mol + 2148.84 mol
= 3760.47 mol
According to ideal gas equation, PV = nRT. Hence, calculate the volume as follows.
PV = nRT
1 atm \times V = 3760.47 mol \times 0.0821 L atm/mol K \times 580 K[/tex] (as
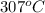 = 307 + 273 = 580 K)
= 307 + 273 = 580 K)
V = 179066.06 L
Thus, we can conclude that total volume of the gas is 179066.06 L.