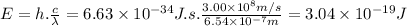Answer:
The energy associated with this transition is 3.04 × 10⁻¹⁹ J.
Step-by-step explanation:
An electron absorbs radiation when it goes from level 2 to level 3. The wavelength associated to this transition can be calculated using Rydberg equation.
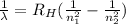
where,
λ is the wavelength of the radiation
RH is the Rydberg constant for Hydrogen (1.10 × 10⁷ m⁻¹)
n₁ and n₂ are the levels (being n₁ < n₂)
In this case,
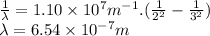
We can calculate the energy associated to this radiation using Planck-Einstein equation.
E = h . ν = h . c / λ
where,
h is the Planck's constant (6.63 × 10⁻³⁴ J.s)
c is the speed of light (3.00 × 10⁸ m/s)
Then,