Answer:
The change in the pH (ΔpH) is 2,17
Step-by-step explanation:
The reaction:
CH₃NH₂(aq) + H₂O(aq) ⇌ CH₃NH₃⁺(aq) + OH⁻
![kb = ([OH^(-)][CH_(3)NH_(3)^+])/([CH_(3)NH_(2)])](https://img.qammunity.org/2020/formulas/chemistry/college/z07bu8cy8rl9l9zek1en1io39ngc6bfbgl.png) (1)
(1)
In equilibrium, a solution of CH₃NH₂ 4,7M produces:
[CH₃NH₂] = 4,7 - x
[CH₃NH₃⁺] = x
[OH⁻] = x
Replacing in (1):
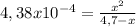
x² + 4,38x10⁻⁴x - 2,0586x10⁻³ = 0
The solutions are:
x = -0,0456 No physical sense. There are not negative concentrations.
x = 0,04515 Real answer.
The concentration of [OH⁻] is 0,04515 M.
As pOH = -log [OH⁻] And pH+pOH = 14. The pH of this solution is:
pH = 12,65
The addition of 6,7M produce this changes in concentrations:
[CH₃NH₂] = 4,656 + x
[CH₃NH₃⁺] = 6,74515 - x
[OH⁻] = 0,04515 - x
Replacing in (1) you will obtain:
x² - 6,7907x + 0,3025 = 0
Solving for x:
x = 6,74586 No physical sense
x = 0,04484 Real answer.
Thus, [OH⁻] = 0,04515 - 0,044842 = 3,08x10⁻⁴M
pOH = 3,51.
pH = 10,49
Thus ΔpH is 12,65 - 10,49 = 2,16 ≈ 2,17
I hope it helps!