Step-by-step explanation:
According to the given data, we will calculate the following.
Half life of lipase
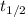 = 8 min x 60 s/min
= 8 min x 60 s/min
= 480 s
Rate constant for first order reaction is as follows.
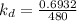
=
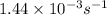
Initial fat concentration
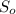 = 45
= 45
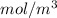
= 45 mmol/L
Rate of hydrolysis
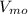 = 0.07 mmol/L/s
= 0.07 mmol/L/s
Conversion X = 0.80
Final concentration (S) =
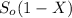
= 45 (1 - 0.80)
= 9
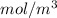
or, = 9 mmol/L
It is given that
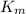 = 5mmol/L
= 5mmol/L
Therefore, time taken will be calculated as follows.
t =
![-(1)/(K_(d))ln[1 - (K_(d))/(V){K_(M) ln ((S_(o))/(S)) + (S_(o) - S)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/6lyzjvnn5r5ff3oo79hzkftorur03h8sim.png)
Now, putting the given values into the above formula as follows.
t =
![-(1)/(K_(d))ln[1 - (K_(d))/(V){K_(M) ln ((S_(o))/(S)) + (S_(o) - S)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/6lyzjvnn5r5ff3oo79hzkftorur03h8sim.png)
=
![-(1)/(1.44 * 10^(-3)s^(-1))ln[1 - (1.44 * 10^(-3)s^(-1))/(0.07 mmol/L/s ){K_(M) ln ((45 mmol/L )/(9 mmol/L )) + (45 mmol/L - 9 mmol/L )]](https://img.qammunity.org/2020/formulas/chemistry/high-school/7xc70jq035epq6jxbiif018jtsa22ayn2v.png)
=
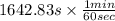
= 27.38 min
Therefore, we can conclude that time taken by the enzyme to hydrolyse 80% of the fat present is 27.38 min.