Answer: Option (C) is the correct answer.
Step-by-step explanation:
The given data is as follows.
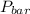 = 745 torr
= 745 torr
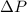 = - 50 (the value is negative because on the open side its level is lower than the side connected to the gas)
= - 50 (the value is negative because on the open side its level is lower than the side connected to the gas)
Now, formula to calculate the pressure of neon gas will be as follows.
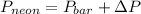
Putting the given values into the above formula as follows.
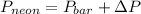
=
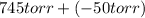
= 695 torr
Thus, we can conclude that the neon pressure is 695 torr.