Answer:
The mass of water required is, m = 1.492 Kg
The final temperature of water,
 = 100 °C
= 100 °C
Step-by-step explanation:
Given that
Energy absorbed by water, q = 4.5 x 10⁵ J
The initial temperature of water
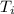 = 25 °C
= 25 °C
The mass of water required to absorb the energy, m = ?
The final temperature of water,
 = ?
= ?
The formula for energy absorbed by the water,
q = m C ΔT Joules
where,
C - specific heat capacity (4200 J/Kg °C)
ΔT =
 -
-
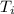 (change in temperature)
(change in temperature)
The maximum possible temperature of the water at Standard Temperature and Pressure is 100 °C
As the final temperature is not given, let us consider the maximum possible temperature of the water.
Therefore,
ΔT =
 -
-
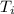
= 100 -25
= 75 °C
To find the mass of water, from the above relation
m = q/CΔT Kg
Substituting the values in the above equation
m = 4.5 x 10⁵/4200 x 75
m = 1.492 Kg
Hence, the mass of water required is m = 1.492 Kg