Answer:
250.756 moles He
Step-by-step explanation:
From the question we are given;
Volume, L = 685 L
Temperature, T = 621 K
Pressure, P = 189 × 10 kPa
We are required to calculate the number of moles of the gas,
Using the Ideal gas equation,
PV = nRT, where P is the pressure, V is the volume, T is the temperature, n is the number of moles, and R is the ideal gas constant.
We can replace the known variables and constant in the equation to get the unknown variable, n.
Using ideal gas constant as 8.3145 L.kPa/K/mol
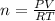
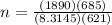
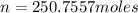
n = 250.756 moles
The moles of helium contained in the sphere is 250.756 moles