Answer:
c. AgNO₃(aq) + NH₄Cl(aq) ->AgCl(s) + NH₄NO₃(aq)
Step-by-step explanation:
We have to follow the solubility rules to check the states of each compound.
-) All the cholride salts are soluble EXCEPT those also containing: lead, silver or mercury.
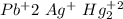
-) All the nitrate
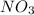 and ammonium salts
and ammonium salts
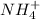 are soluble.
are soluble.
With this in mind
 ,
,
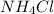 and
and
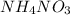 would be soluble, so we have to use the aqueosus state (aq).
would be soluble, so we have to use the aqueosus state (aq).
The
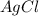 then would have a solid state (s).
then would have a solid state (s).