Answer:
ΔK = 3.57 eV We can see this is less than the energy needed to ionize the atom so the process does not occur
Step-by-step explanation:
In order to respond we must know what the energy of the atoms is after the shock. We create a system formed by the two atoms, whereby the amount of movement is conserved
p₀ = m v₀
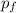 = (m + M) v
= (m + M) v
p₀ =
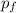
v = m / (m + M) v₀
Let's look for the initial velocity of the oxygen atom
K = ½ m v₀²
v₀ = √2K / m
We calculate the final system speed
v = m / (m + M) (√ 2K/m)
We found the final energy
Kf = ½ (m + M) v2
Kf = ½ (m + M) [m² / (m + M)² 2K/m]
Kf = m/(m + M) K
Let's replace and calculate
Kf = 16u / (16u + 133u) K
u = 1.67 10⁻²⁷ kg
Kf = 16/149 K
Kf = 0.107 K
Kf = 0.107 4.0
Kf = 0.428 eV
This is the kinetic energy of the system after the collision, what remains is the energy available for ionization
ΔK = Ko -Kf
ΔK = 4 - 0.428
ΔK = 3.57 eV
We can see this is less than the energy needed to ionize the atom so the process does not occur