Answer:
Empirical formula of the compund is C₆H₇SNO₂
Step-by-step explanation:
The moles produced of CO₂ are the same than moles of C and the moles of H₂O are half moles of H.
4,23 g CO₂ ×
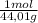 = 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
= 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
1,01 g H₂O ×
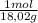 = 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
= 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
Moles of SO₃ are the same than moles of S and moles of HNO₃ are the same than moles of N.
2,11 g SO₃ ×
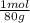 = 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
= 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
2,27 g HNO₃ ×
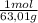 = 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
= 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
wt% of Oxygen is 100-9,07-20,43-4,49-45,80 = 20,21 wt%
With wt% it is possible to obtain relative moles dividing in molar mass of each atom thus:
moles of C: 45,80/12,01 = 3,81
moles of H: 4,49/1,01 = 4,44
moles of S: 20,43/32,05 = 0,64
moles of N: 9,07/14 = 0,65
moles of O: 20,21/16 = 1,26.
Dividing in the least number:
C: 3,81/0,64 = 6
H: 4,44/0,64 = 7
S: 0,64/0,64 = 1
N: 0,65/0,64 = 1
O: 1,26/0,64 = 2
Thus, empirical formula of the compound is:
C₆H₇SNO₂
I hope it helps!