The mass of the copper block is 37.82g
To determine the mass of the copper block, we can use the principle of conservation of energy, specifically the heat transfer equation:
![\[ q_{\text{copper}} = q_{\text{water}} \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/xf8xu802ny3gideajtb2xar84q957od3ue.png)
The heat gained or lost (q) can be calculated using the formula
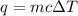 , where m is the mass, c is the specific heat capacity, and
, where m is the mass, c is the specific heat capacity, and
 is the change in temperature.
is the change in temperature.
For copper:
![\[ q_{\text{copper}} = m_{\text{copper}}c_{\text{copper}}\Delta T_{\text{copper}} \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/io0f590x27ky3vuvbm54ipcet9tfgz4dr0.png)
For water:
![\[ q_{\text{water}} = m_{\text{water}}c_{\text{water}}\Delta T_{\text{water}} \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/z6a8ks0mfoapi3lra3gi4g5autk1pc4z1f.png)
At thermal equilibrium, the heat gained by the copper is equal to the heat gained by the water:
![\[ m_{\text{copper}}c_{\text{copper}}\Delta T_{\text{copper}} = m_{\text{water}}c_{\text{water}}\Delta T_{\text{water}} \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/l4ss5hp5ku71qlzshpjzf22sshh7usztwn.png)
Rearrange the equation to solve for the mass of the copper block
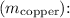
![\[ m_{\text{copper}} = \frac{m_{\text{water}}c_{\text{water}}\Delta T_{\text{water}}}{c_{\text{copper}}\Delta T_{\text{copper}}} \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ja8clea13a06slwnr7f3nhsd6f295at9de.png)
Substitute the given values and solve:
![\[ m_{\text{copper}} = \frac{(95.7 \, \text{g})(4.18 \, \text{J/g}^\circ \text{C})(24.2^\circ \text{C} - 22.7^\circ \text{C})}{(0.385 \, \text{J/g}^\circ \text{C})(65.4^\circ \text{C} - 24.2^\circ \text{C})} \]](https://img.qammunity.org/2020/formulas/chemistry/high-school/wedopmp6gjlu9p844e6z1tr680xk7vf83o.png)
Therefore, The mass of the copper block is 37.82g