Answer:
27.03 %
Step-by-step explanation:
The formula for the calculation of moles is shown below:

Given: For calcium
Given mass = 10.0 g
Molar mass of calcium = 40.078 g/mol
Moles of calcium = 10.0 g / 40.078 g/mol = 0.2495 moles
According to the given reaction:
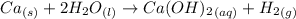
1 mole of calcium on reaction forms 1 mole of calcium hydroxide
Thus,
0.2495 moles of calcium on reaction forms 0.2495 mole of calcium hydroxide
Moles of calcium hydroxide = 0.2495 moles
Molar mass of calcium hydroxide = 74.093 g/mol
Thus, Mass = Moles * Molar mass = 0.2495 moles * 74.093 g/mol = 18.5 g
Theoretical yield = 18.5 g
Given experimental yield = 5.00 g
% yield = (Experimental yield / Theoretical yield) × 100 = (5.00/18.5) × 100 = 27.03 %